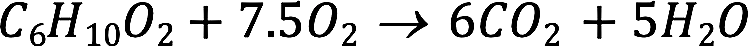top of page
Isomers, Spectroscopy and the Ideal Gas Equation
2017 Paper Two
Question Ten
Looking at past AQA A-level Chemistry questions, I wanted to go beyond the answers and focus on thought processes, ideas and tips that will help in examinations.
The series will help you to spot weaknesses and help with revision, or it can serve as addenda to your notes.If you haven't gone through the paper, please look up the questions at www.aqa.org.uk, or click the direct link below, and have a go...
AQA A-level CHEMISTRY Paper 2 Organic and Physical ChemistryMonday 19 June 2017
Data Booklet here
A systematic approach is needed here...
A 5-carbon chain numbered from the functional group lying on the same side (zame = Z) before and after the double bond (-ene). Methyl group on C2. This gives us...
Z-2-methylpent-2-ene-1-oic acid
Common errors include incorrect rearrangement of the equation, missing conversions for volume, temperature and pressure and the answer left as grams at the end. All these marks were cheaply lost in the original exam.
The presence of the gas constant always makes me think of the ideal gas equation, PV = nRT - the appearance of V, P and T confirms this.
You should look through the question and think "are there any conversions needed?' - clearly there are:
Pressure must be in Pascals = 105 000
Temperature in Kelvin = 298
Volume in cubic metres (we'll convert that next)
The equation for the combustion is...
Balanced...
The volume of carbon dioxide 'consumed' by passing over NaOH...
335 - 155 = 180 cubic centimetres = 0.000180 cubic metres.
rearranging gives us...
Put the values .into the equation...
From the balanced equation, the ratio of carboxylic acid to carbon dioxide is 1:6
∴ moles of carboxylic acid...
Nearly there...
The mass of the carboxylic acid is...
Done
First thing - latch on to the fact that the compound is cyclic. This narrows down the choices with only 6 carbons to choose from.
In the IR spectrum, there is a peak around 2500-3000 indicating the presence of an OH (from an acid), C=O at 1750. In the C-13 NMR, there are 4 peaks indicating the carbons are in 4 distinct environments and the chemical shift to just above 180 indicates a carbonyl
The diagram shows the 4 different environments for the carbons.
The carboxyl group will take one carbon leaving 5 carbons for a cyclic structure - a pentagon. The carboxyl takes account of the two oxygens. Hydrogen count is good for this structure
Common issues:
Spectra are not always dealt with well by students - spectroscopy is usually the last subject taught at A-Level and it may be that limited time issues could be having an effect.
A number of students failed to state that the IR absorption (between 2500 and 3000) showed an O−H bond in an acid.
Double-check that you are reading the correct chemical shifts
The thing about these isomers is that they both display symmetry...
Both R and S have three peaks - 3 distinct carbon environments.
In the first molecule, R, there are 4 carbons next to C=O
S has 2 carbons next to C=O in range 𝛿 = 20 - 50 R will have two peaks, S only one in this range
Both isomers will have 2 peaks - for R, these will be singlets (two identical CH2 groups); S will show a triplet and a quartet
Make sure you get as much practice as possible analysing spectra
This is a condensation
You could place the -O- on the other side
DO NOT enclose in brackets
It's important to show the trailing bonds - do not enclose the repeating unit in brackets.
There are strong, non-polar C-C bonds that cannot be hydrolysed.
bottom of page