top of page
Vanadium Compounds and Ions
2018 Paper One
Question Nine
7 mark question.
1. The most common incorrect answer was Zn2+.
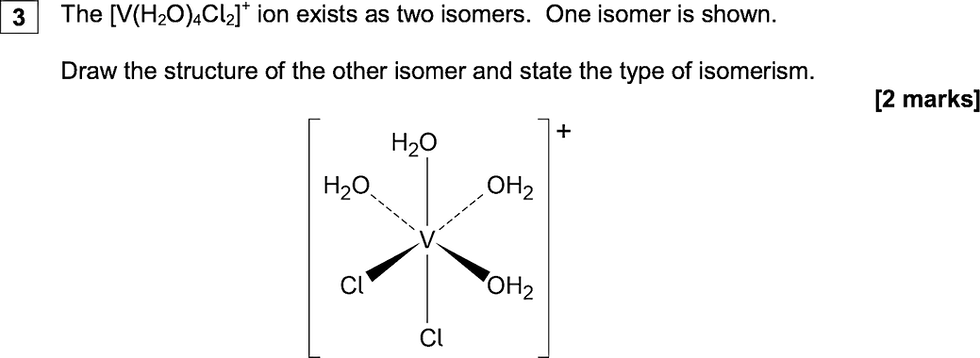
3. The structure was often given, incorrectly, as a mirror image and the type of isomerism as optical;
4. Incorrect equations included charged vanadium species.
bottom of page